DaS EDC
- According to GAMP5 system verification, the system is safe and reliable
- Complies with the electronic signature requirements of FDA 21 CFR Part 11
- Complies with regulations such as ICH GCP and NMPA
- Flexible database structure to meet different clinical trial requirements
- Multilingual version, supporting global multicenter clinical research development
- Highest standard IDC computer room in China for remote backup to ensure data security
- Strong technical team develops the system; Operation and Maintenance Engineer 7 × 24-hour rapid response, providing professional technical support.

Efficient and Easy to use
Humanized interface UI layout and functional design, enabling quick and precise switching of various modules, reducing user usage costs and improving efficiency;
Standardized
Provide standardized data reports and manage team data from different dimensions; Support CDISC standard ODM.xml import and export.
Flexible
Support site, subject, visit, form, and field level operation, reduce repetitive system operations;
Platformized
Platform integration management module for research creation, research center management, coding configuration, and data sharing settings;
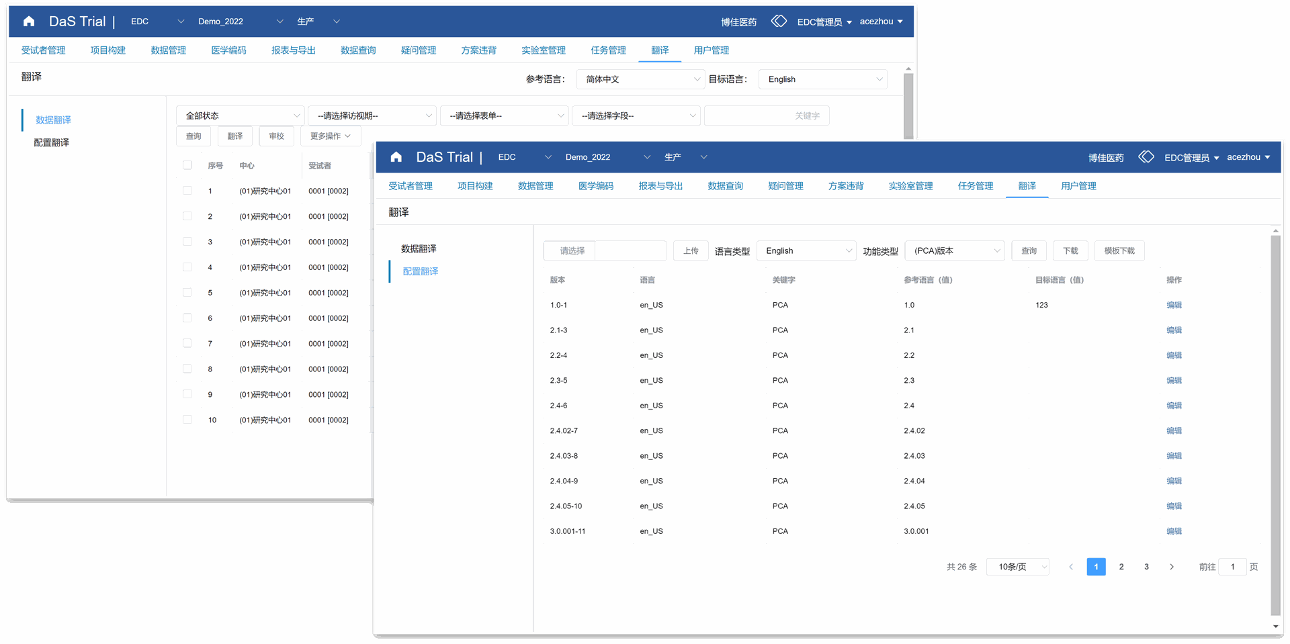
Translation Workbench
Support configuration and subject data translation, and provide a complete solution for data translation
Online or offline translation selection; Translation and proofreading process ensures data accuracy
Data Standardization
Support ODM.xml import and export of CIDSC standard
System API for data exchange between different supplier systems; Including Subject data and Admin data, perform database configuration reading and updating based on Admin data

Rich applied Experience
- More than 3000 clinical trials have been conducted using DaS EDC for data collection and management, covering Phase I-IV new drug registration and national research projects, applied to various indications.
- More than 500000 subjects were collected and applied to many national major special researches, such as AIDS prevention and treatment, HPV database; Support the Shanghai three-year action plan database platform.
- DaS EDC has been used in over 800 hospitals/institutions, covering the entire china (including Hong Kong and Taiwan).
- Serving over 600 enterprises and research institutions, and also participating in international multi center experiments, with rich experience.