BioVoice&BioGuider
Company Profile
国内知名的临床研究数统技术服务的专业化公司,均为国家级高新技术企业和专精特新企业。公司由业内知名专家领衔,拥有稳定的专业团队,全面完善的质量体系,丰富的监管沟通经验,项目经验覆盖广泛适应症领域,多年持续深耕积淀形成的“工具平台 + 技术服务 + 模型引导”一体化全流程解决方案,已成为其独特工作模式,成功助力多个药物获批上市,深得广大客户信任和尊敬。
Integrated platform
公司拥有临床试验方法学工具产品的强大研发团队,涉及医学、药学、数学与统计学、计算机与软件科学等众多专业,自主研发了数智化临床试验数据平台(DaS Trial®)和AI赋能的建模模拟平台(Maspectra®)。
DaS Trial®平台包括EDC、IWRS、PVS、ePRO、Insight、eConsent、Imaging、RSM、eTMF等功能,平台化管理避免数据多方采集,为临床试验高质量数据提供保障。
Maspectra®平台包括PK、BE、PK-PD等功能,为模型引导的药物研发(MIDD)实施必备工具,助力临床研发精确高效推进。
All Process services
公司基于自主研发的两大平台系统提供配套技术服务,服务范围包括随机盲法、数据管理、生物统计、临床药理、定量药理、医学事务、系统评价、药物警戒等。
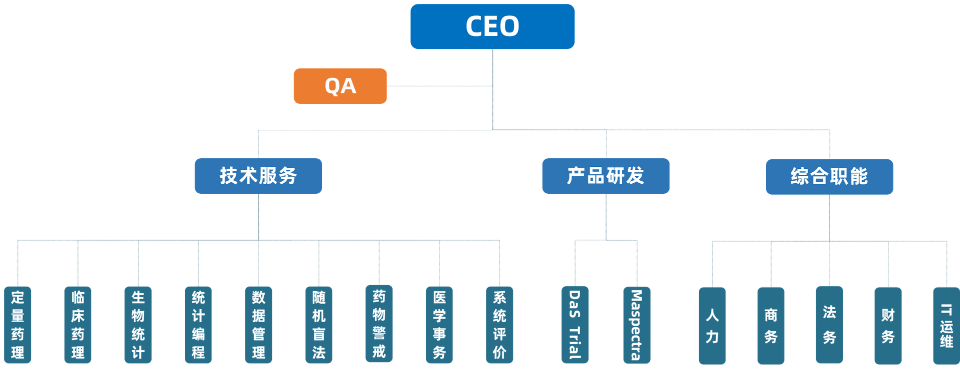
Organizational Structure
History
MaS V2.0 publish
DaS Trial publish
MaS V1.0 publish,PV 2.0 publish
BioVoice(Hefei) Incorporation
PV V1.0 publish
Third generation EDC system publish
Second generation EDC system publish
BioGuider Incorporation
First generation EDC system publish
BioVoice Incorporation